Question
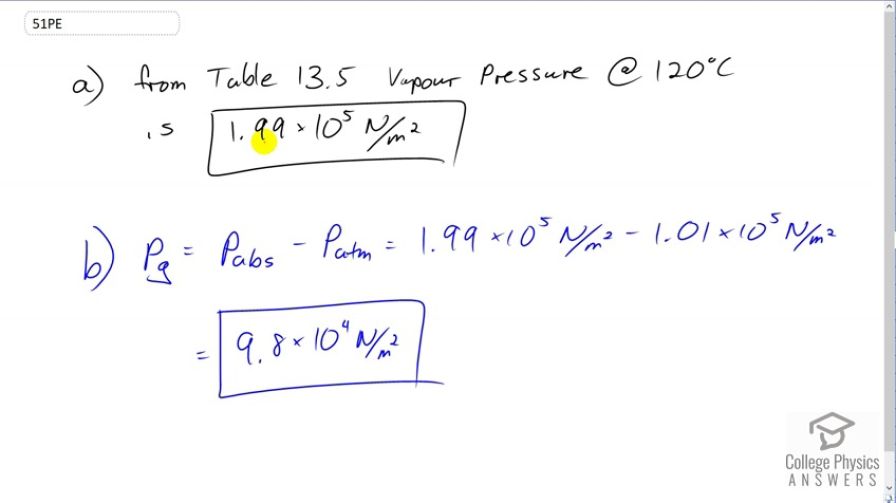
Pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. (a) What pressure is necessary to raise the boiling point to ? (b) What gauge pressure does this correspond to?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 51 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Looking at Table 13.5 at 120 degrees Celsius, we can see that the vapor pressure for water is 1.99 times ten to the five newtons per square meter. And so, it's this pressure that is needed to cause boiling to occur at 120 degrees Celsius. Now, the gauge pressure is this absolute pressure minus atmospheric pressure. And so with 1.99 times ten to the five newtons per square meter minus 1.01 times ten to the five newtons per square meter giving a gauge pressure of 9.8 times ten to the four newtons per square meter.
