Question
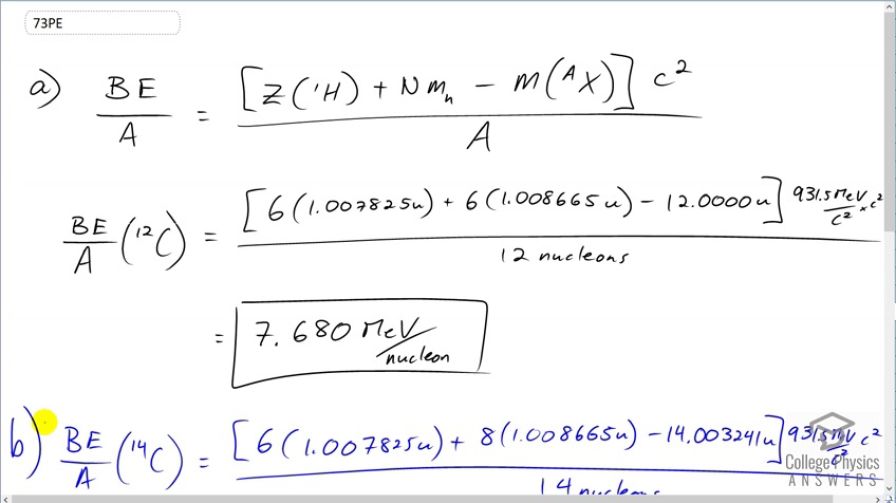
(a) Calculate BE / A for . Stable and relatively tightly bound, this nuclide is most of natural carbon. (b) Calculate BE / A for . Is the difference in BE / A between and significant? One is stable and common, and the other is unstable and rare.
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 73 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to calculate the binding energy per nucleon of carbon-12 in part (a) and then for carbon-14 in part (b). So binding energy is the number of protons multiplied by the atomic mass of hydrogen plus the number of neutrons in the nuclide times the mass of a bare neutron minus the atomic mass of the nuclide. So we have 6 times 1.007825 atomic mass units; this is the atomic mass of hydrogen including its electron that's attached and we have 6 electrons that we don't want them as I have included in this number and then this mass here for the atomic mass of carbon also includes 6 electrons and the mass of 6 electrons and so that means these 6 electron masses cancel out and so that's good and we are left in the end only with a difference in the nuclides. And so we have 6 times the mass of a neutron here as well. And we convert that into megaelectron volts per c squared and divide by 12 nucleons and we get 7.680 megaelectron volts per nucleon in carbon-12. For carbon-14, the number of protons is the same so this is still 6 here and but we have 8 neutrons instead of 6 and then we have a different atomic mass for carbon-14 and we are dividing by 14 nucleons. and we get 7.520 megaelectron volts per nucleon. It takes less energy to split up the nucleons in a carbon-14 nucleus than it does to split up all the nucleons in a carbon-12 nucleus. Now to see if that difference is significant or not, we'll find the percent difference between them and this is the binding energy per nucleon for carbon-12 minus that of carbon-14 divided by carbon-12's binding energy per nucleon and we get a difference of 2.1 percent. And I guess that's sufficient to make carbon-14 tend to decay more likely than the more tightly bound carbon-12. Since this is more tightly bound meaning that it takes takes more energy to break it apart; the carbon-12 is stable whereas the carbon-14 is not stable since it has less binding energy per nucleon.


