Question
Write the complete decay equation for the given nuclide in the complete notation. Refer to the periodic table for values of Z:
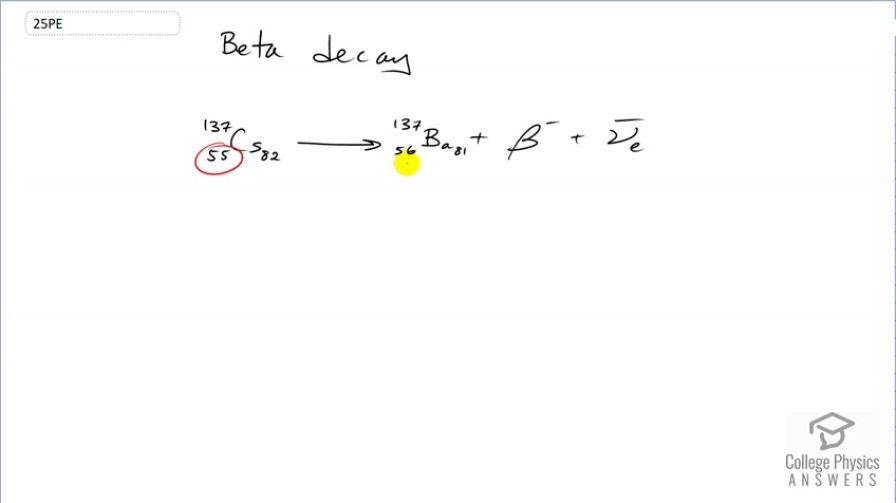
decay producing . The parent nuclide is a major waste product of reactors and has chemistry similar to potassium and sodium, resulting in its concentration in your cells if ingested.
decay producing . The parent nuclide is a major waste product of reactors and has chemistry similar to potassium and sodium, resulting in its concentration in your cells if ingested.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 25 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. The β-decay of caesium-137 results in an increase in the number of protons from 55 to 56 as a result of one of these neutrons turning into a proton and an electron. So as the number of protons increases from 55 to 56, that's an increase in positive charge of 1 and so there needs to be a compensating negative charge created in order for charge to be conserved on both sides. So on the left side, we have a positive charge of 55 and on the right hand side, we have a positive charge of 56 minus the 1 of this electron or β-particle and so for a total of 55 on the right hand side. So charge is conserved so that's good. The number of nucleons or more generally, the conservation of baryon number is also upheld by there being 137 nucleons on both sides. And there's also this rule about the conservation of electron family number. Now by creating this electron in order to conserve charge, we are also causing a change in the electron family number of positive 1 with the presence of this particle. And on the left side, we had zero; there's no electrons there floating around and so there needs to be a another particle having an electron family number of negative 1 which this electron anti-neutrino has so that there's a total of zero electron family number on the right hand side here as well.