Question
A physicist scatters rays from a substance and sees evidence of a nucleus in radius. (a) Find the atomic mass of such a nucleus. (b) What is unreasonable about this result? (c) What is unreasonable about the assumption?
Final Answer
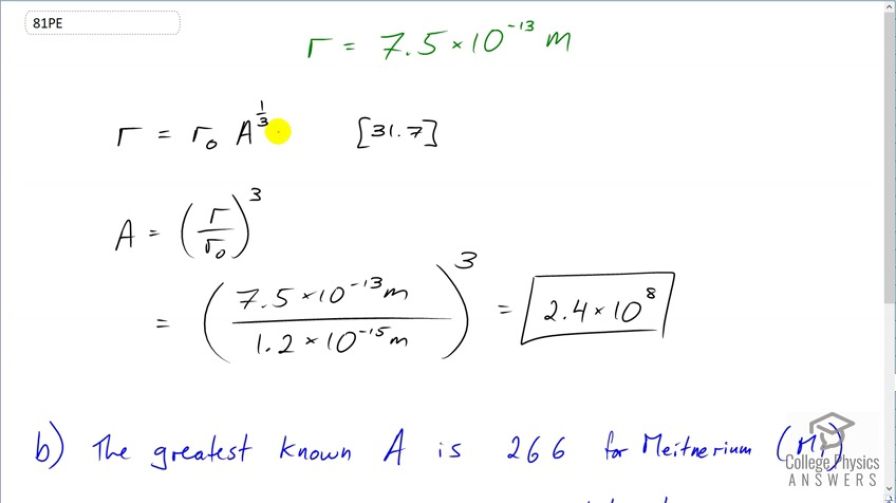
- The greatest known mass number is 266 for Meitnerium (Mi). $2.4\times 10^{8} is 6 orders of magnitude larger, and therefore unrealistic.
- The measurement can not be assumed to be the nuclear radius.
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 81 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Let's find the mass number of a hypothetical nucleus with a radius of 7.5 times 10 to the minus 13 meters. Here's a formula number 7 from chapter 31 which says that the radius of a nucleus is r naught times the mass number to the power of one-third. We can divide both sides by r naught and then raise both sides to the power of 3 and then switch the sides around and we solve for the mass number. So it's the radius divided by r naught to the power of 3. r naught is 1.2 times 10 to the minus 15 meters and the radius we are allegedly observing here in this question is 7.5 times 10 to the minus 13 meters; cube that and you get a mass number of 2.4 times 10 to the 8. Now that is very unreasonable because the largest known mass number is 266 for meitnerium and this number here is six orders of magnitude larger than the largest ever observed. So that's not going to be possible. And so the measurement that's being taken here is not the radius of the nucleus.
