Question
Confirm that charge, electron family number, and the total number of nucleons are all conserved by the rule for decay given in the equation . To do this, identify the
values of each before and after the decay.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 31 (Problems & Exercises)
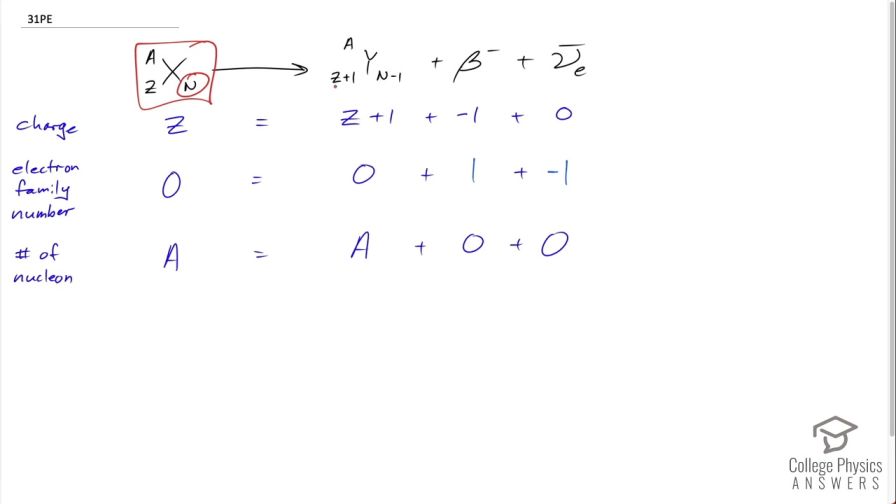
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. In beta decay, a neutron and the parent nuclide turns into a proton and an electron so this β-particle is another way of saying electron and the daughter nuclide will have one additional proton compared to the parent nuclide so we have Ƶ plus 1. Let's check each of these conservation rules to make sure they are all followed in this process. So we check conservation of charge: on the left hand side, we have a total charge of Ƶ protons so positive Ƶ and on the right hand side, we have Ƶ plus 1 from the daughter nuclide but we have a compensating negative 1 charge from this β-particle that's produced as well and the electron anti-neutrino has no charge and so on the right hand side, this total is also Ƶ so that checks out and we have Ƶ equals Ƶ. For electron family number, there are no particles on the left side that have an electron family number and so the left side is 0; on the right hand side, it's 0 for the daughter nuclide and it's positive 1 for the β-particle and negative 1 for the electron anti-neutrino and so the total on the right hand side is 0 equaling the total on the left, which is also 0 so that checks out. And the number of nucleons: on the left side, we have A nucleons— that's Ƶ plus N— and on the right hand side, it's the same number of nucleons Ƶ plus 1 plus N minus 1 so in other words, it's Ƶ plus N as well. Same number of nucleons, the β-particle and the electron anti-neutrino do not have any nucleons at all and so it's A equals A and that checks out too.