Question
Write the complete decay equation for the given nuclide in the complete notation. Refer to the periodic table for values of Z:
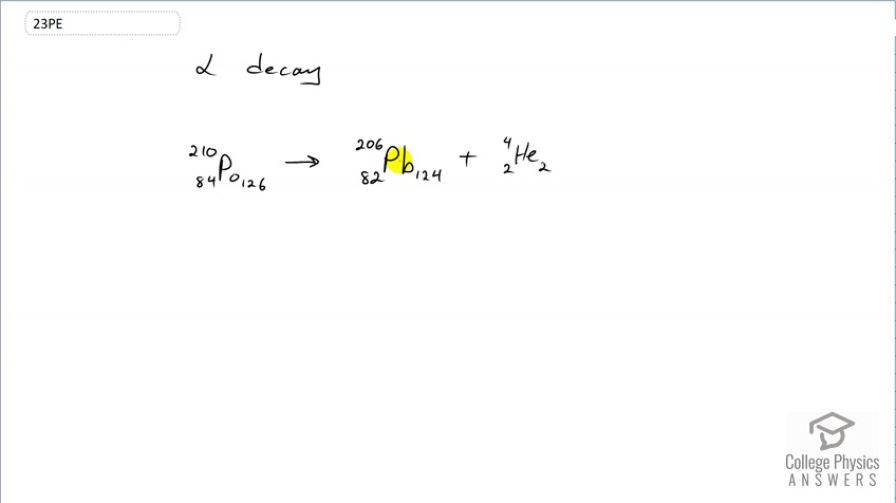
decay of , the isotope of polonium in the decay series of that was discovered by the Curies. A favorite isotope in physics labs, since it has a short half-life and decays to a stable nuclide.
decay of , the isotope of polonium in the decay series of that was discovered by the Curies. A favorite isotope in physics labs, since it has a short half-life and decays to a stable nuclide.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 23 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. The α-decay of pulonium 210 into lead 206 and an α-particle is written like this where you have an α-particle consisting of two protons bound with two neutrons. So the number of protons in the pulonium gets decreased by 2 from 84 to 82 and 82 is the atomic number of lead. And the number of neutrons decreases from 126 to 124 and those two protons and neutrons are contained in this α-particle.