Question
You can smell perfume very shortly after opening the bottle. To show that it is not reaching your nose by diffusion, calculate the average distance a perfume molecule moves in one second in air, given its diffusion constant to be
.
Final Answer
1 mm
Solution video
OpenStax College Physics, Chapter 12, Problem 62 (Problems & Exercises)
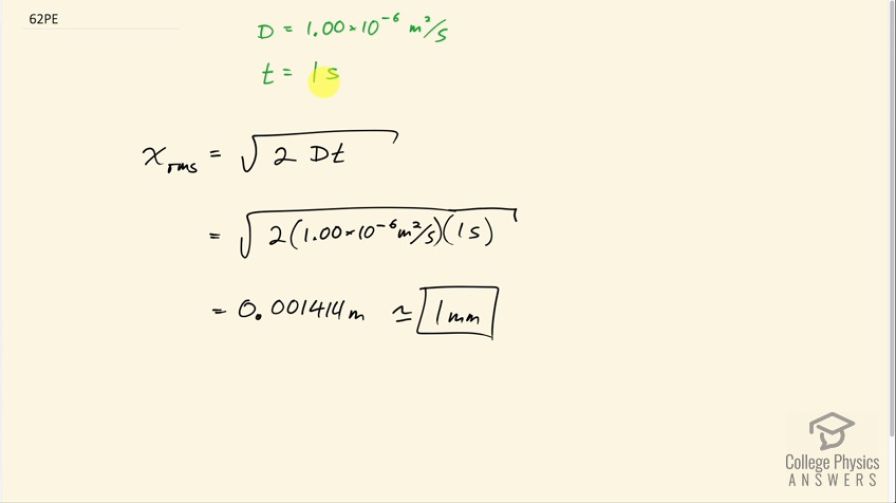
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We're going to show that perfume does not reach your nose within one second as a result of diffusion because we're going to show that the distance it travels due to diffusion is very small. The diffusion constant for these perfume molecules in air is 1.00 times 10 to the minus six meters squared per second. And the time we're interested in is one second. And the sort of average, the root mean squared distance that we would expect a perfume molecule to travel is square root of two times the diffusion constant times time. So, that’s square root of two times one times 10 to the minus six meters squared per second times one second, which is about one millimeter. And so the reason the perfume reaches in nose quickly is because it's been sprayed or somehow pushed through the air.
