Question
(a) Calculate the energy in eV of an IR photon of frequency . (b) How many of these photons would need to be absorbed simultaneously by a tightly bound molecule to break it apart? (c) What is the energy in eV of a ray of frequency ? (d) How many tightly bound molecules could a single such γ ray break apart?
Final Answer
Solution video
OpenStax College Physics, Chapter 29, Problem 26 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
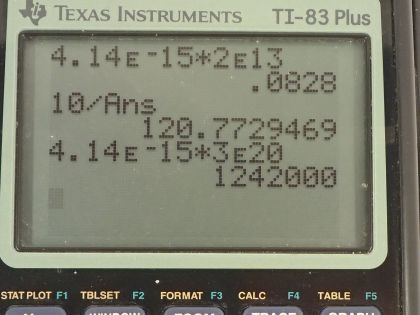
This is College Physics Answers with Shaun Dychko. We are going to calculate the energy in electron volts of an infrared photon that has a frequency of 2.00 times 10 to the 13 hertz; the energy is Planck's constant times the frequency so that's 4.14 times 10 to the minus 15 electron volt seconds times the frequency where I have chosen Planck's constant to have these units because that's convenient since the seconds here will cancel with the reciprocal seconds that are implicit in the hertz unit— hertz is 1 over seconds— so these seconds cancel with these seconds and we are left with electron volts, which is what the question asks for so that's 0.0828 electron volts. Part (b) says how many of these photons would be needed to get absorbed simultaneously by a tightly bound molecule to break it apart? So table [29.1] tells us the amount of energy holding together a tightly bound molecule and the energy then is 10 electron volts so that's the total energy needed from however many number of photons that we are going to calculate here. So the total energy is going to be the number of photons multiplied by the energy per photon and then we can solve for N by dividing both sides by the energy per photon. So the number of photons then is 10 electron volts divided by 0.0828 electron volts per photon and that gives us 121 photons needed. In part (c), we want to find the energy of a 3.00 times 10 to the 20 hertz gamma ray so we multiply that by Planck's constant in units of electron volt seconds and that is 1.24 times 10 to the 6 electron volts and then in part (d), we are asked how many tightly bound molecules could a single gamma ray break apart? So the energy of this single photon is going to equal the number of molecules broken apart multiplied by the energy in total per molecule to break it so we'll divide both sides by E total and we have the number of molecules then is the energy per photon divided by the energy needed to break apart each molecule; this letter N here is different from the letter N up here, you will see that this fraction is flipped over compared to the fraction down here the ends represent different things... this is the number of molecules. So this is 1.242 times 10 to the 6 electron volts per photon for the gamma ray photon divided by 10 electron volts needed to break apart each molecule and this works out to molecules per photon and that is 1.24 times 10 to the 5 molecules per photon. So this tells you how destructive a gamma ray can be because a single photon can break apart 10 to the 5 molecules whereas with infrared photons, you would need 121 of them to break apart one single molecule. So gamma rays can be very destructive and that's why they are implicated in things like cancer and so on.